Which gas is usually liberated when an acid reacts with a metal? Explain with a suitable example.
Subject
Science
Standard
Class 12
Views
1851
Asked By
Anaisha

Answer / Solution
Hydrogen gas is liberated when an acid reacts with a metal. For example, when zinc metal reacts with dil.
HCL Hydrogen is evolved and salt zinc chloride is formed as:
Zn (g) + 2HCL (aq) → ZnCl2 (aq) + H2 (g)
To test the presence of H2 gas, bring a burning splinter near the mouth of the test tube where H2 gas is released, the match stick bums with a pop sound.

Answer / Solution
Mostly, hydrogen gas is usually librated when an acid reacts with a metal.
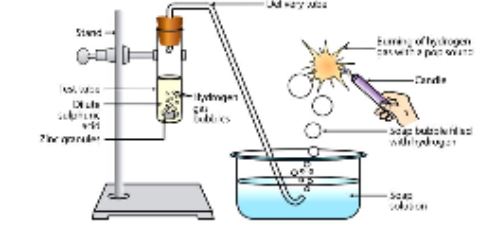
First, take the two pieces of zinc granules in the test tube and dil H2SO4 to it. Shake it and pass the gas produced into a soap solution. After that, the bubbles of soap solution are formed. These soap bubbles contain hydrogen gas.
The CHEMICAL EQUATION is of reaction is
H2SO4 + Zn → ZnSO4 + H2
Identification of as testis, if we burning candle near the soap bubbles, it will burn with a soap sound.
Top Trending Questions
- 9114 Views Thomson’s model of an atom, explain how the atom is neutral as a whole.
- 5109 Views What are the main characteristics of peer to peer systems?
- 4666 Views Draw a plot of α-particle scattering by a thin foil of gold to show the variation of the number of the scattered particles with scattering angle.
- 3706 Views Difference between Synchronous and Asynchronous Transmission
- 3482 Views Explain protocols in details.
- 3427 Views Explain advantages and disadvantages of mobile computing.
- 3319 Views Differentiate between homogeneous and heterogeneous mixtures with examples
- 3062 Views Define olfactory indicators. Name two subtances which can be used as olfactory indicator
- 3058 Views Properties of the Real Number System
- 2935 Views The first term of an AP is 5, the last term is 45 and the sum is 400. Find the number of terms and the common difference.
- 2745 Views What are canal rays? Why anode rays are called canal rays. Mention two postulates of J.J. Thomsons Model
- 2709 Views What is the difference between interactive mode and script mode in Python?
- 2699 Views Different Data Mining Tools in details
- 2612 Views Explain various Access Modifiers in Java
- 2584 Views How do you download files using ftp?
- 2579 Views What type of information is provided by MIS and DSS?
- 2521 Views What is ftp? How do you use ftp?
- 2510 Views What do you mean by data archiving?
- 2507 Views What is animation? How are animated video clips generated? Also, What are the applications of computer graphics?
- 2490 Views What Is data mining? What is the role of data archiving in data mining?
- 2486 Views Adaptability and adaption in mobile computing.
- 2483 Views What is a text-to-speech system? What are its applications?
- 2474 Views What type of creative works are given copyright? What is the period of a copyright?
- 2460 Views What is a mainframe? What are the good and bad characteristics of a mainframe?
- 2459 Views What is a recordable CDROM? How does it work?
- 2411 Views Ramkali saved Rs 5 in the first week of a year and then increased her weekly saving by Rs 1.75. If in the nth week, fher weekly savings become Rs 20.75, find n.
- 2403 Views Where is the Sahara desert located?
- 2394 Views What do you understand by aerodynamically floating head?
- 2392 Views What is speech recognition? What are its applications?
- 2315 Views What is voice mail? What are the advantages and disadvantages of voice mail?
- 2312 Views Explain the design steps of the transform mapping.
- 2312 Views Explain different types of Data Mining Techniques.
- 2304 Views What is the basic difference between transaction processing system and MIS?
- 2291 Views What is the difference between transaction processing and on-line transaction processing
- 2260 Views Write the distribution of electrons in carbon and sodium atoms
- 2244 Views Necessary conditions for autotrophic nutrition
- 2228 Views What Are the Different Types of Machine Learning?
- 2221 Views Write first four terms of the A.P. when the first term a and the common difference d are given as follows
- 2213 Views What is a DVDROM? What is its typical capacity?
- 2199 Views How do you ensure information integrity?
- 2160 Views When will you say a body is in Uniform acceleration and non-uniform acceleration?
- 2158 Views Explain Atomic number, Mass number, Isotopes and Isobars with some examples. Also give any two uses of isotopes.
- 2143 Views Write advantages and disadvantages of Hierarchical Data Model
- 2125 Views How are water and minerals transported in plants?
- 2113 Views What does an Electrical Circuit Mean, Electrical Current and its units.
- 2110 Views What is telnet? How is it used?
- 2097 Views A sum of Rs 700 is to be used to give seven cash prizes to students of a school for their overall academic performance. If each prize is Rs 20 less than its preceding prize, find the value of each of the prizes.
- 2094 Views What is SRS? Why is it important? How does one arrive at a SRS?
- 2080 Views Which term of the AP: 21, 18, 15, . . . is – 81? Also, is any term 0? Give reason for your answer.
- 2078 Views The houses of a row are numbered consecutively from 1 to 49. Show that there is a value of x such that the sum of the numbers of the houses preceding the house numbered x is equal to the sum of the numbers of the houses following it. Find this value of x. [Hint : Sx-1 = S49 – Sx ]
- 2061 Views What is feasibility analysis? How is it used?
- 2056 Views What are the factors which influence the adsorption of a gas on a solid?
- 2055 Views Naresh earns 30800 per month. He keeps 50% for household expenses. what he save per month
- 2051 Views Calculate the molecular masses of the following elements, Hydrogen, Oxygen, Chlorine, Carbon Di Oxide
- 2051 Views Write a note on civilizing mission of the colonisers and Huynh Phu So
- 2043 Views 60 percent of the students in a school are girls, If the total number of girls in the school is 690
- 2028 Views 1500 families with two children are selected randomly according to the data given. Compute the probability of a family, chosen
- 2022 Views What is rational number
- 2009 Views Define parallel connection and series connection?
- 2008 Views What is the role of desorption in the process of catalysis?
- 2006 Views What are the activities carried out during system implementation phase?
- 1998 Views Why did James Mill and Thomas Macaulay think that European education was essential in India?
- 1981 Views Explain how data mining may be used by a State Road Transport Corporation
- 1971 Views Nested Loops in C
- 1965 Views What are the different parts of a URL? Explain the purpose of each part
- 1963 Views Explain in detail about Tableau in data visualization?
- 1954 Views What rights does copyright give to the owner of the copyright?
- 1937 Views How will you find the valency of chlorine, sulphur and magnesium?
- 1923 Views What are polyatomic ions? Give examples?
- 1915 Views Describe the ideas behind the Tonkin Free School. To what extent was it a typical example of colonial ideas in Vietnam.
- 1913 Views Class 8 Quiz MCQ Basic Questions
- 1911 Views The sum of the third and the seventh terms of an AP is 6 and their product is 8. Find the sum of the first sixteen terms of the AP.
- 1895 Views A long solenoid with 15 turns per cm has a small loop of area 2 cubic cm placed inside the solenoid to its axis.
- 1886 Views What’s the difference between a generative and discriminative model?
- 1877 Views looping structure in python
- 1871 Views What is meant by Intellectual Property Rights (IPRs)?
- 1829 Views In a cricket match, a batsman hits a boundary 6 times out of 30 balls she plays. Find the probability that she did not hit a boundary.
- 1806 Views 2 cubes each of volume 64 cm3 are joined end to end. Find the surface area of the resulting cuboids.
- 1801 Views What is open source software? List some open source software.
- 1796 Views How is our atmosphere different from the atmospheres on Venus and Mars?
- 1773 Views Difference between Artificial intelligence and Machine learning
- 1768 Views What is system evaluation? Why is it required?
- 1768 Views Connect python to mysql database
- 1767 Views What is the syntax of Java Inheritance? Explain with example.
- 1766 Views Explain the inference rules for functional dependencies in DBMS
- 1722 Views Distinguish between speed and velocity.
- 1708 Views What is data Mining.
- 1703 Views What is Internet telephony? In what way is it different from Internet radio?
- 1697 Views If the sum of the first n terms of an AP is 4n − n2, what is the first term (that is S1)? What is the sum of first two terms?
- 1693 Views What is a Trojan? In what way is it different from a virus? How do you remove a Trojan from your PC?
- 1647 Views Explain why, when a motorcar makes a sharp turn at a high speed, we tend to get thrown to one side.
- 1645 Views What is a mobile computing?
- 1601 Views What is Internet radio? What are the advantages and disadvantages of Internet radio.
- 1599 Views Distinguish between the meaning of the terms adsorption and absorption. Give one example of each.
-
1598 Views
Check whether the following is quadratic equation:
(x + 1)2 = 2(x – 3) - 1584 Views What is Inheritance in Java? Explain.
- 1579 Views Why did a major protest erupt in 1926 in the Saigon Native Girls School in Vietnam ? Explain.
- 1576 Views How soil is formed?
- 1560 Views What is vector graphics? Where are vector graphics used?
- 1549 Views To counter the Chinese influence what steps did the French take in the sphere of education?
- 1549 Views feature of mobile computing
- 1538 Views Achievements of the Hindustan socialist Republican Association
- 1534 Views How do you know that your PC has been infected by a virus? If it is infected. how do you remove the infection?
- 1532 Views What is a computer virus? How does it infect computer systems?
- 1532 Views What is the difference between MIS and DSS?
- 1527 Views The sum of 4th and 8th terms of an A.P. is 24 and the sum of the 6th and 10th terms is 44. Find the first three terms of the A.P.
- 1519 Views What is plagiarism? Is copyright violation a criminal offence?
- 1494 Views What is a thin client? Why are thin clients preferred in certain applications?
- 1494 Views Give an example of client-server computing
- 1486 Views Types of data in mobile computing
- 1479 Views What are the three types of intellectual property? What kind of rights does IPR on computer software normally give?
- 1457 Views Shweta obtained 18 marks in a test of 25 marks. What was her percentage of marks
- 1451 Views How do you ensure security of your database?
- 1440 Views Data Mining History
- 1436 Views List some IT related products which are given patent rights and trademarks.
- 1431 Views What is mathematics
- 1422 Views What is information integrity? How is it different from data security?
- 1419 Views Two spherical bobs, one metallic and the other of glass, of the same size are allowed to fall freely from the same height above the ground
- 1400 Views What is data security? What is the difference between data privacy and security?
- 1369 Views What do you understand by image generation? What are the two types of geometrical objects which can be generated using a computer?











